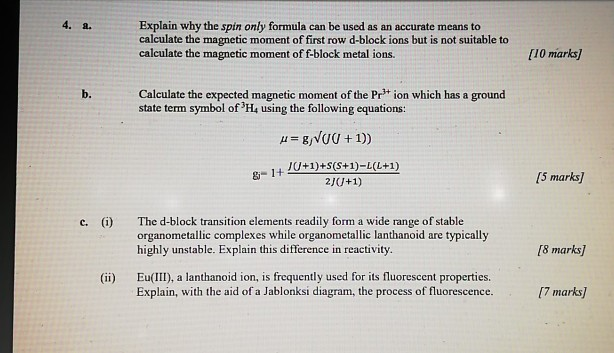
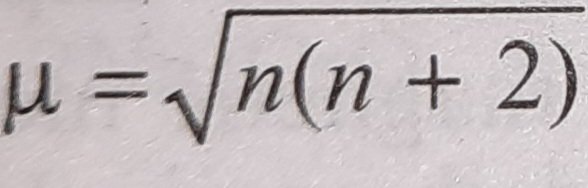56.Spin only magnetic moment of Mnx+ ion is root 15B.M.Then what is tge value of X OPTIONS:A)6 B)4 C)2 D)8
20. which of following have almost equal spin only and observed magnetic moment a)Fe(II) b)Ni(II) c)Cu(II) d)V(II)

58. If spin only magnetic moment of vcl, is 1.73 BM then correct formula is :- * vci, (2) VCI, X (3) VCI, (4) VCI, 53 -1.732. 3ds socarz?

Write the name of two metal which used in maximum composition in mischmetal. Calculate the value of the magnetic moment of V^{+2}

58. If spin only magnetic moment of vcl, i 1.73 BM then correct formula is :- (1) VCI, (2) VCI, (3) VCI, (4) VCI,

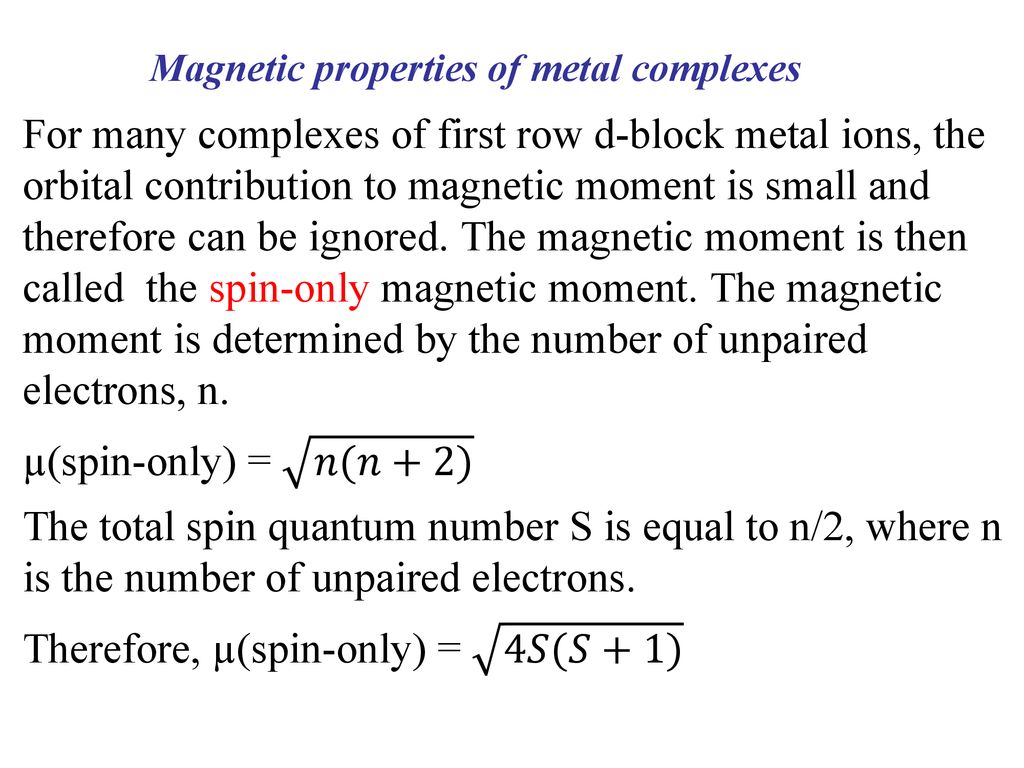
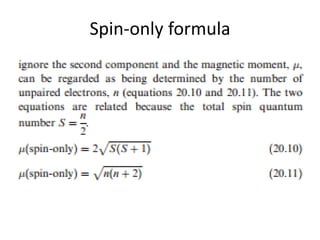
ReasonThe spin only magnetic moment of an ion is equal to sqrt {n(n+2)} where n is the number of unpaired electrons in the ion.AssertionThe spin only magnetic moment of Sc^{3+} is 1.73
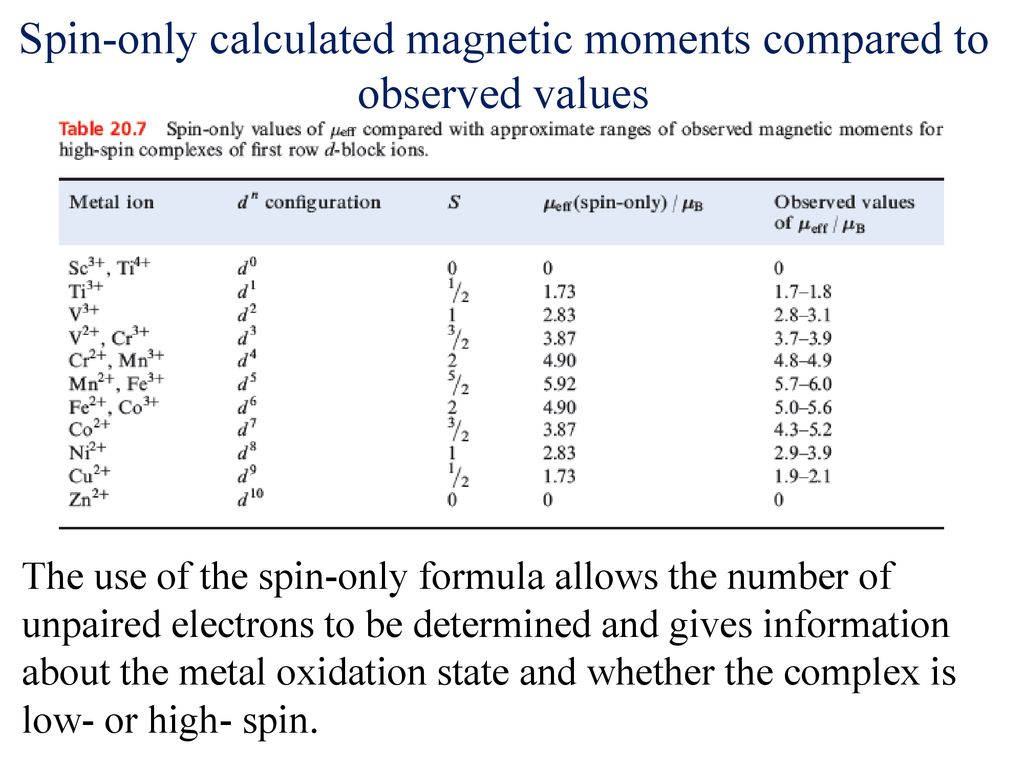
![SOLVED: The spin-only formula to calculate the magnetic moment of a compound is: p = [N(N+2)]^(1/2) * uB where N is the number of unpaired electrons and uB is the Bohr magneton. SOLVED: The spin-only formula to calculate the magnetic moment of a compound is: p = [N(N+2)]^(1/2) * uB where N is the number of unpaired electrons and uB is the Bohr magneton.](https://cdn.numerade.com/ask_images/8bd8e65e68104e63897b868130353014.jpg)

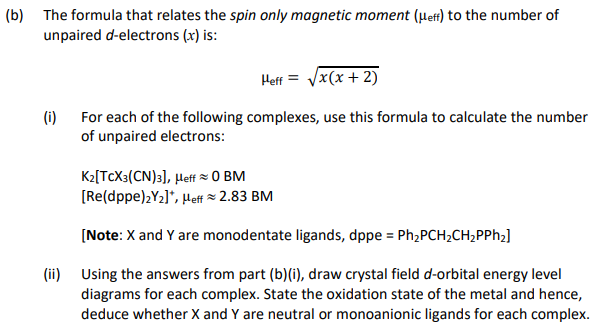
![The spin-only magnetic moments of k\u0104[fe(oxalate),] and k,[ru(oxalate),] are The spin-only magnetic moments of k\u0104[fe(oxalate),] and k,[ru(oxalate),] are](https://cdn.eduncle.com/library/scoop-files/2020/7/image_1594373088294.jpg)


![The magnetic moment (spin only) of [NiCl4]2– is - askIITians The magnetic moment (spin only) of [NiCl4]2– is - askIITians](https://files.askiitians.com/cdn1/cms-content/common/www.askiitians.comonlinetestforumsimages204-1316_sataug1615-54-35.jpg.jpg)
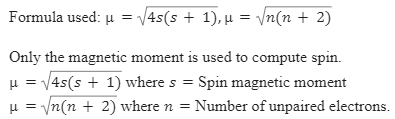

![Assamese] What is 'spin only' formula? Assamese] What is 'spin only' formula?](https://d10lpgp6xz60nq.cloudfront.net/ss/web-overlay-thumb/8950532.webp)